Pristine Surgical announces the addition of Christopher Cleary to its Board of Directors

Pristine Surgical, a medical device company dedicated to simplifying endoscopy with its 4K single-use digital arthroscopes, today announced the addition of Christopher Cleary to its Board of Directors. Chris recently served as Senior Vice President of Corporate Development for Medtronic, where he led acquisition and investment strategies, including the landmark $50 billion acquisition of Covidien in 2014.
Director, Electromechanical Systems and Optical Engineering (remote)
The Director, Electromechanical Systems and Optical Engineering is responsible for setting and leading the global teams and managing all electrotechnical systems development including hardware and firmware for the Pristine product platforms.
Pristine Surgical announces full market release of Summit™ 4K single-use surgical arthroscope across the U.S.

Pristine Surgical, a medical device company dedicated to making endoscopy more efficient, consistent, and safe, has accelerated the launch of its Summit™ 4K single-use surgical arthroscope—the first of its kind. After an overwhelmingly positive response during its limited market release, Summit™ is now available to hospitals and ambulatory surgery centers across the United States.
Pristine Surgical completes first live arthroscopy using a 4K single-use surgical arthroscope at Southern California Orthopedic Institute (SCOI)

Pristine Surgical – a medical device company that combines single-use endoscopes with cloud-based software to make minimally invasive visualization more efficient, consistent, and safe – completed its first in human procedure using Summit™, the world’s first 4K single-use surgical arthroscope.
AAOS 2023 was an important moment in our journey to simplifying endoscopy

Image quality, hand-feel, and having a brand-new scope for every case – these are some of the things the orthopedic community heralded in their feedback to us after experiencing Summit™ at the American Association of Orthopedic Surgeons (AAOS) annual meeting in Las Vegas March 7-11.
Pristine Surgical Receives FDA 510(k) Clearance for Summit™, the World’s First 4K Single-Use Surgical Arthroscope
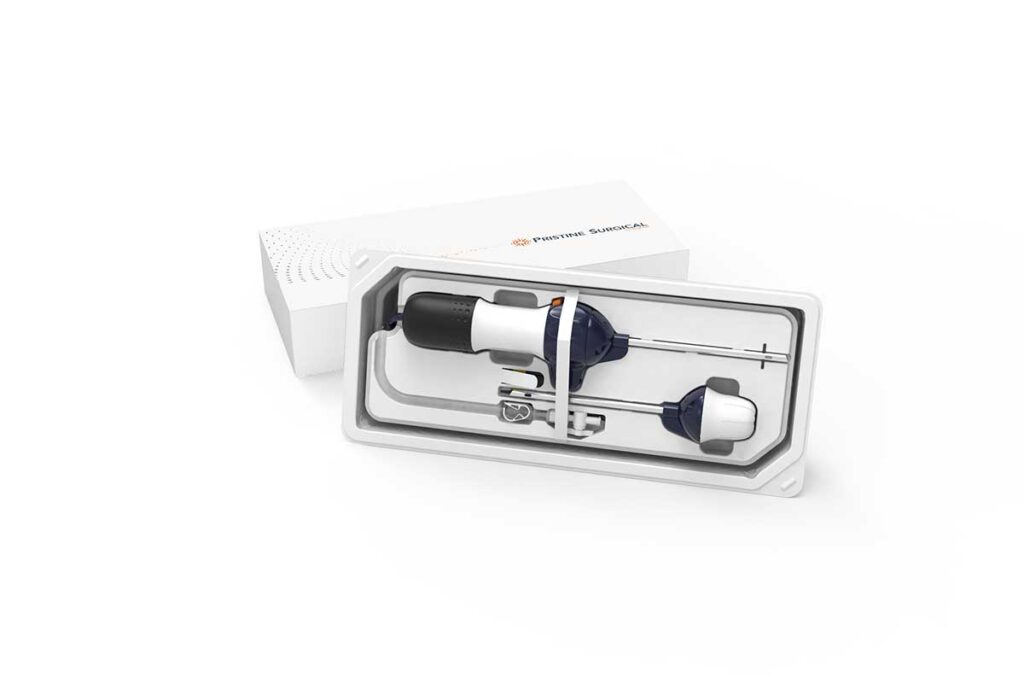
Medical device company Pristine Surgical received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for Summit, a first-of-its-kind 4K single-use surgical arthroscope designed to improve the efficiency, consistency, and safety of arthroscopic procedures.
1099 Sales Representatives
Team Pristine is seeking U.S. Based Distribution Partners to market and support Summit™ – a 4K single-use surgical arthroscope designed to improve the efficiency, consistency, and safety of arthroscopic procedures. Ideal candidates will have distribution experience and existing relationships with ASC’s providing endoscopy services.


